Cardiovascular implants - Endovascular devices - Part 1: Endovascular prostheses - ISO 25539-1
DIN EN ISO 25539-1 – Cardiovascular implants – endovascular devices – Part 1: endovascular prostheses
This standard specifies requirements for the evaluation of endovascular systems (prostheses and delivery systems). It is applicable to endovascular systems used to treat aneurysms, stenoses or other avascular anomalies or pathologies (e.g. dissection, transections) or to create shunts between vessels. Similar prosthesis configurations (e.g. fenestrated, branched) are within the scope, but there is no description for specific requirements.
Tests included are:
- Endovascular system:
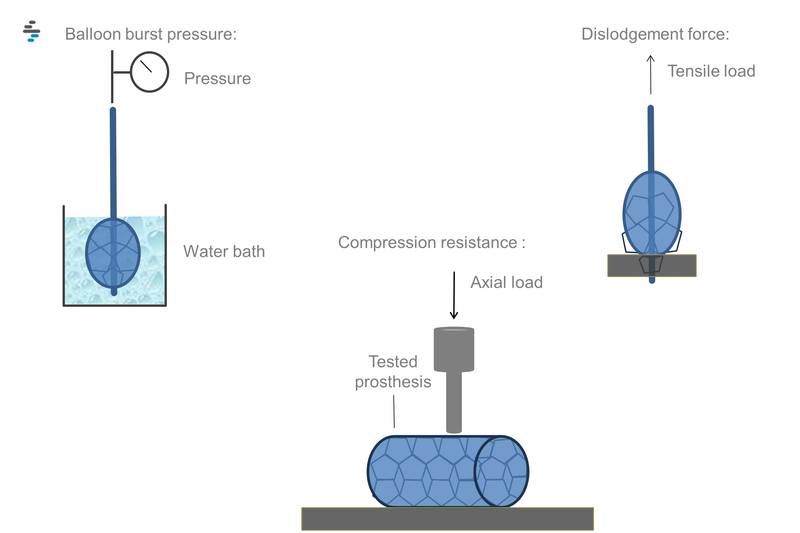
- Balloon testing (Balloon burst pressure, balloon deflation times, balloon rated fatigue, balloon volume to burst)
- Dimensional verification of the endovascular system
- Dislodgement force (pre-mounted, balloon-expandable endovascular prostheses)
- Force to deploy
- Simulated use
- Tensile bond strength
- Torsional bond strength
- Haemostasis
- Biocompatibility
- Sterilization assurance
- Visibility
- Endovascular prostheses
- Corrosion
- Fatigue and durability (radial, active, axial, bending, torsional)
- Fixation and seal (leakage, migration resistance, separation forces)
- Patency-related tests (compression resistance, crush resistance, radial force, resistance to kinking)
- Permeability (integral water leakage, porosity, water entry pressure, water permeability)
- Sizing-related testing (dimensional verification, implant diameter to balloon inflation pressure, implant length to diameter relationship, recoil)
- Strength (burst strength, factory anastomotic strength, longitudinal tensile strength, strength after repeated puncture, strength of the connection of bonds)
- MRI safety