Packaging for terminally sterilized medical devices - Part 1: Requirements for materials, sterile barrier systems and packaging systems - ISO 11607-1
ISO 11607-1 - Packaging for terminally sterilized medical devices — Part 1: Requirements for materials, sterile barrier systems and packaging systems
The ISO 11607-1 standard specifies requirements and test methods for materials, preformed sterile barrier systems, sterile barrier systems and packaging systems that are intended to maintain sterility of terminally sterilized medical devices until the point of use.
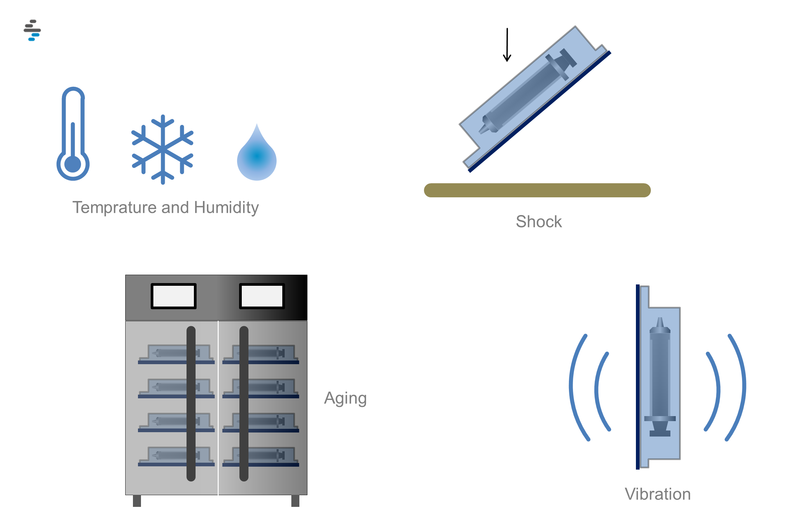
- Packaging system performance testing - the packaging system must provide adequate protection from all hazards of handling, distribution and storage against shock and vibration, compression, temperature, humidity, mode of transport, pressure changes, etc.
- Stability testing - Sterile barrier systems must not lose their integrity over time. Stability testing shall be performed with real-time aging, however, stability testing with accelerated aging protocols shall be considered sufficient evidence of the stated expiration dates until data from real-time aging studies are available.