WELCOME TO SPINESERV - Accredited Implant and Medical Device Testing Services
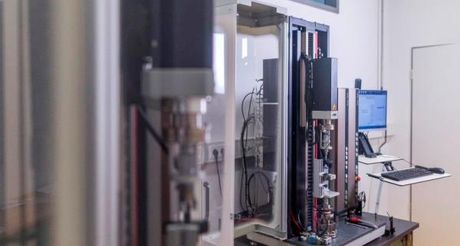
Since 2007 we are specialized on the mechanical testing of implants, medical devices, surgical instruments and implant materials. We test according to standards (ISO, EN, DIN, etc.) or develop and validate customized, individual solutions adapted to the requirements of your medical devices.
Our Know-How - Your Success - as one of the foundation pillars of our corporate philosophy clearly defines our mission to create the best conditions for safe and reliable test results through the interaction of science and technology.
Our Quality - Your Safety - SpineServ GmbH & Co. KG is a DIN EN ISO/IEC 17025 accredited testing laboratory. Maintaining and continuously developing our accreditation is one of our most important goals.
OUR SERVICE PORTFOLIO - Implants and Medical Devices
PRODUCT TYPES
-
Spinal Implants
-
Endoprostheses (Hip, Knee, Shoulder, Further Implants)
-
Osteosynthesis Implants
-
Dental Implants
-
Medical Devices (Connectors, Drug Delivery Systems, Syringes and Needles, Catheters)
-
Packaging
-
Other Medical Devices (Stents, Instruments, Auxiliary Devices)
-
Implant Materials
-
Surgical Instruments
TEST CATEGORIES
- ASTM, ISO or other recognized standards
- Customized solutions that specifically address your requirements
- Static testing / Dynamic fatigue testing
- Wear testing
- Corrosion testing
- Compression / Tension / Torsion / Multiaxial testing
- Leakage / Flow / Kink / Drop testing
- Testing during the development process
- Testing for approval
- Testing required for clinical complaint management
SUPPORTING PROCEDURES
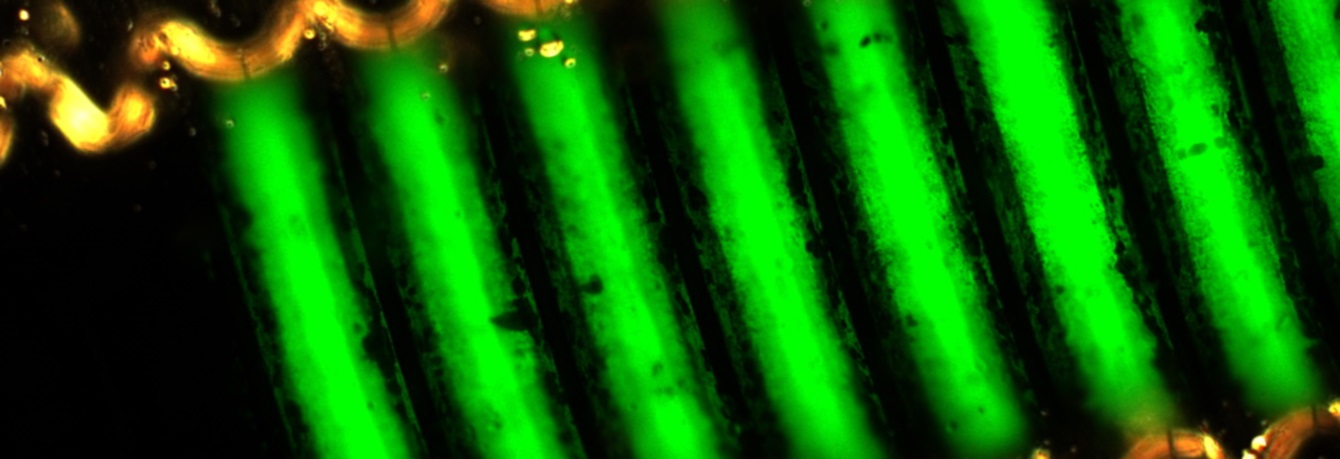
Implants and implant materials can be subjected to accompanying analytical procedures at SpineServ, such as optical surface and particle analysis
Accreditation DAkkS
SpineServ GmbH & Co. KG is accredited according to DIN EN ISO/IEC 17025:2018, which is an accreditation accepted by the FDA.
- accredited test methods (PDF-Download)
- accreditation certificate (PDF-Download)

Accreditation A2LA - Mechanical Testing
SpineServ GmbH & Co. KG is additionally accredited by the American accreditor A2LA according to ISO/IEC 17025:2017.
- accreditation certificate and scope of accreditation (PDF-Download)